Diamonds are a special crystallisation form of carbon atoms. Carbon is nature’s principal building block: plants, animals and mankind too consist of carbon compounds. Diamond crystals came into existence about 3 billion years ago, at a depth of 140 to 200 kilometers inside the earth; they originated from carbon which was exposed to a very high pressure (up to 70,000 kg per cm2) at a very high temperature (up to 2,000 degrees Celcius).
About 5% of the total world production of diamonds is processed into jewelry diamonds: the rest is used for industrial purposes. Since the industrial revolution in the 19th century, diamonds, on account of their hardness, have had many applications: polishing discs, drills, chisels, but also in electronic appliances for drawing extremely thin and precise conducting wire, or in very precise medical instruments.
In some industrial applications for diamonds, they are used up and have to be replaced with new ones regularly. So there is an ongoing demand. Since the fifties of last century, it is possible to produce artificial diamonds. Nowadays, 95 – 99% of diamonds used in industry are synthetic.

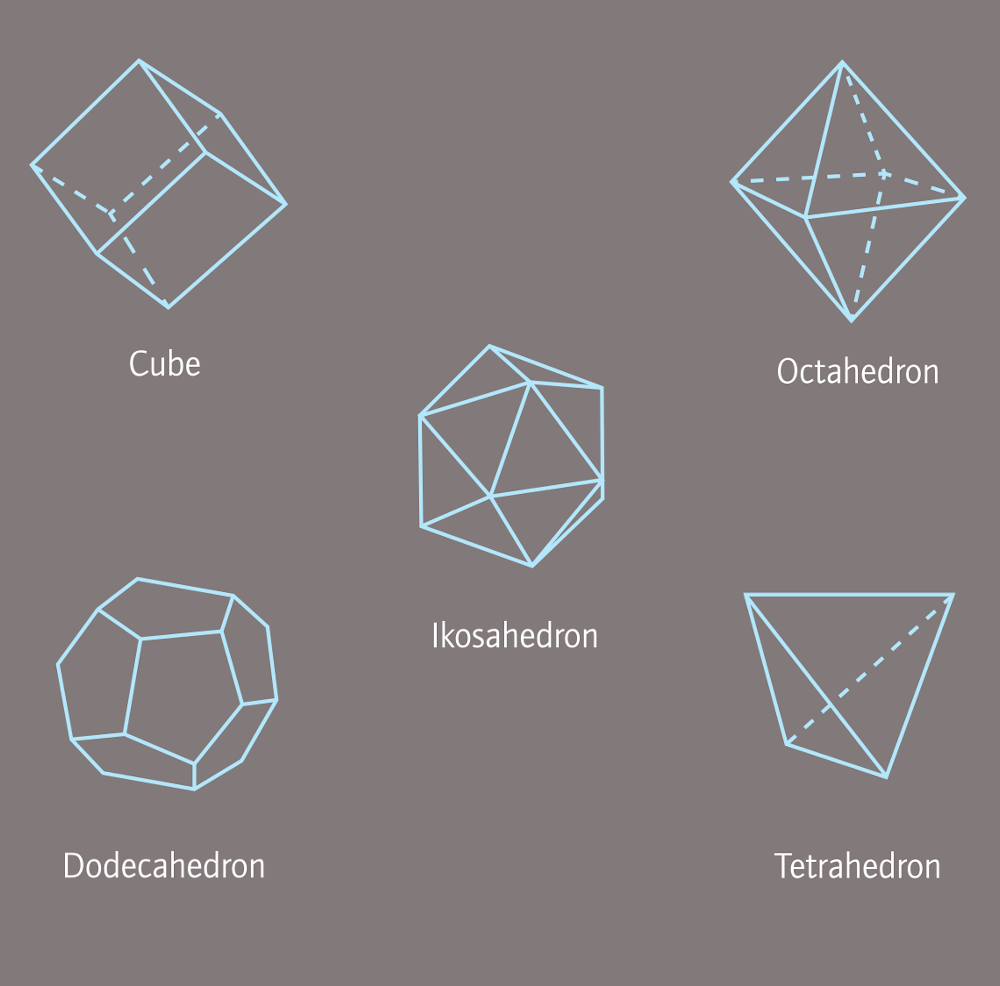
Like the double four-sided pyramid (octahedron), that is considered one of the most perfect forms to process. Crystalisation appears frequently in nature, for example: ice-crystal, rock-crystal or other gemstones.
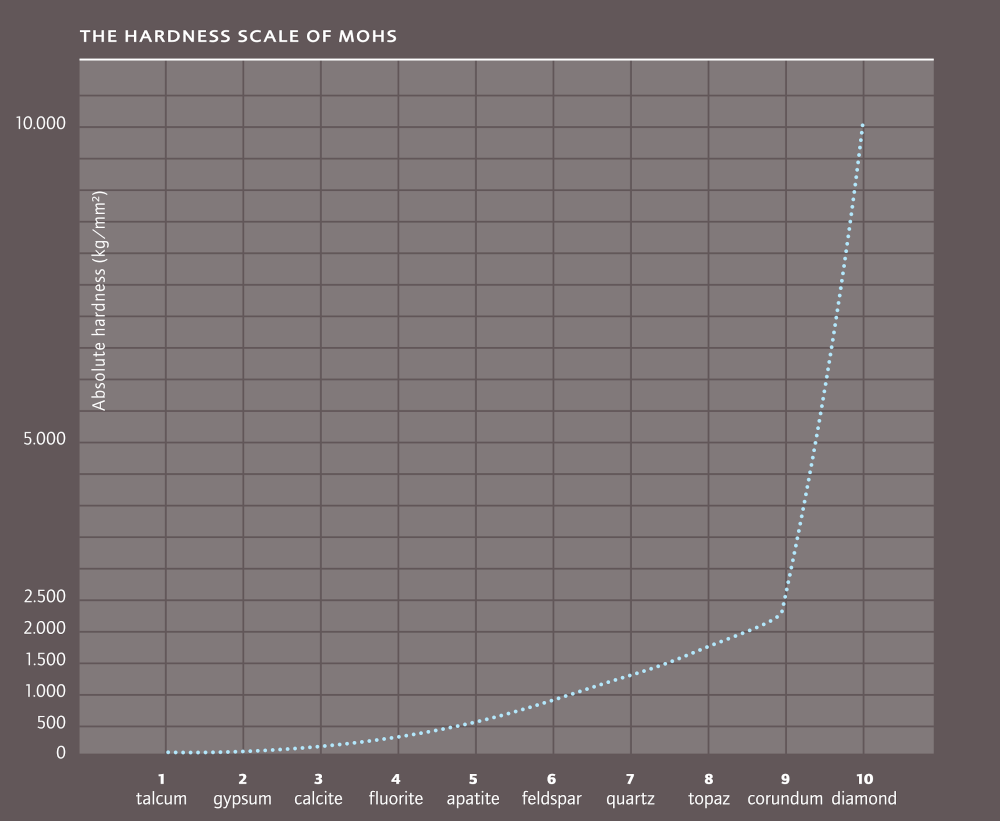
In 1822 the Austrian mineralogist Friedrich Mohs compiled a hardness scale for minerals. In 10 stages, running from soft to very hard. At the bottom is talc (1) at the top, diamond (10).
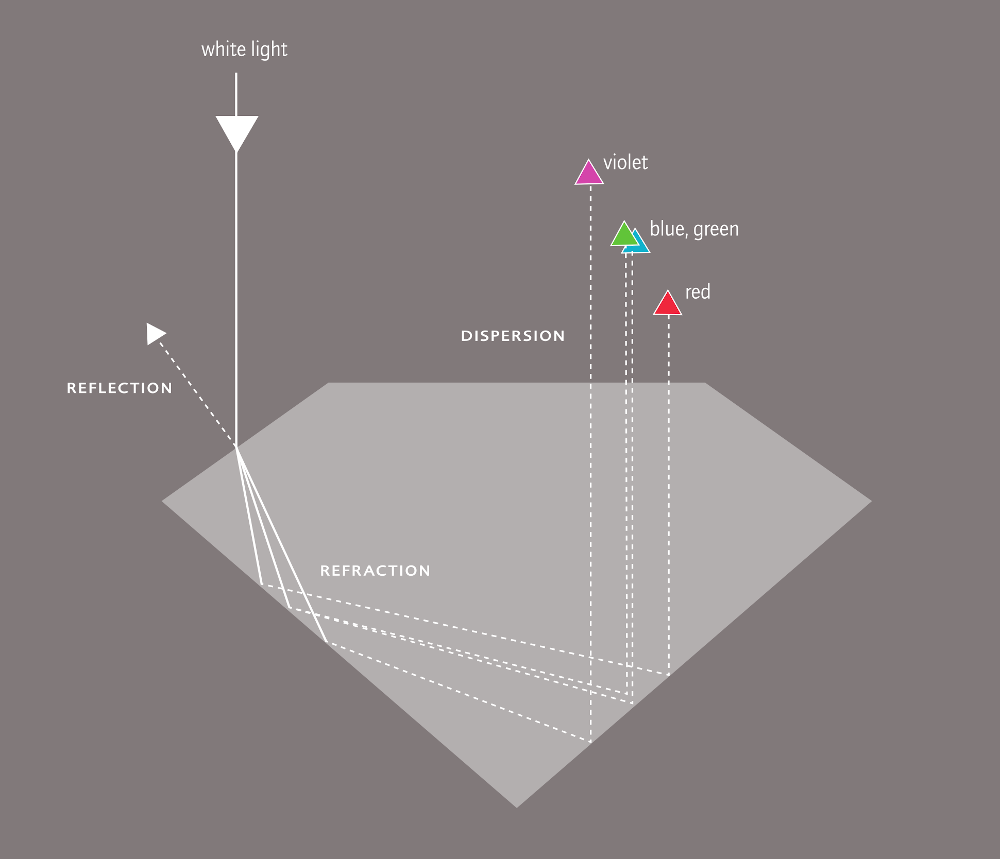
In cut diamonds, light is captured and reflected as in no other precious stone. The effect of reflection and refraction is called brilliance, the play of colours as result of dispersion is called fire. Sparkle or life is mentioned in connection with the light effect that arises when the diamond is moved in light.
About 5% of the total world production of diamonds is processed into jewellery diamonds: the rest is used for industrial purposes. Since the industrial revolution in the 19th century, diamonds, on account of their hardness, have had many applications: polishing discs, drills, chisels, but also in electronic appliances for drawing extremely thin and precise conducting wire, or in very precise medical instruments. In some industrial applications for diamonds, they are used up, and have to be replaced with new ones regularly. So there is an ongoing demand. Since the fifties of last century it has been possible to produce artificial diamonds. Nowadays 95 - 99% of diamonds used in industry are synthetic.
| Cookie | Duration | Description |
|---|---|---|
| __cfduid | 1 month | The cookie is used by cdn services like CloudFare to identify individual clients behind a shared IP address and apply security settings on a per-client basis. It does not correspond to any user ID in the web application and does not store any personally identifiable information. |
| _GRECAPTCHA | 5 months 27 days | This cookie is set by Google. In addition to certain standard Google cookies, reCAPTCHA sets a necessary cookie (_GRECAPTCHA) when executed for the purpose of providing its risk analysis. |
| cookielawinfo-checbox-analytics | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Analytics". |
| cookielawinfo-checbox-functional | 11 months | The cookie is set by GDPR cookie consent to record the user consent for the cookies in the category "Functional". |
| cookielawinfo-checbox-others | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Other. |
| cookielawinfo-checkbox-advertisement | 1 year | The cookie is set by GDPR cookie consent to record the user consent for the cookies in the category "Advertisement". |
| cookielawinfo-checkbox-necessary | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookies is used to store the user consent for the cookies in the category "Necessary". |
| cookielawinfo-checkbox-performance | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Performance". |
| csrftoken | 1 year | This cookie is associated with Django web development platform for python. Used to help protect the website against Cross-Site Request Forgery attacks |
| elementor | never | This cookie is used by the website's WordPress theme. It allows the website owner to implement or change the website's content in real-time. |
| viewed_cookie_policy | 11 months | The cookie is set by the GDPR Cookie Consent plugin and is used to store whether or not user has consented to the use of cookies. It does not store any personal data. |
| Cookie | Duration | Description |
|---|---|---|
| _ga | 2 years | This cookie is installed by Google Analytics. The cookie is used to calculate visitor, session, campaign data and keep track of site usage for the site's analytics report. The cookies store information anonymously and assign a randomly generated number to identify unique visitors. |
| _gat_gtag_UA_167950907_1 | 1 minute | This cookie is set by Google and is used to distinguish users. |
| _gid | 1 day | This cookie is installed by Google Analytics. The cookie is used to store information of how visitors use a website and helps in creating an analytics report of how the website is doing. The data collected including the number visitors, the source where they have come from, and the pages visted in an anonymous form. |
| _hjFirstSeen | 30 minutes | This is set by Hotjar to identify a new user’s first session. It stores a true/false value, indicating whether this was the first time Hotjar saw this user. It is used by Recording filters to identify new user sessions. |
| Cookie | Duration | Description |
|---|---|---|
| _fbp | 3 months | This cookie is set by Facebook to deliver advertisement when they are on Facebook or a digital platform powered by Facebook advertising after visiting this website. |
| fr | 3 months | The cookie is set by Facebook to show relevant advertisments to the users and measure and improve the advertisements. The cookie also tracks the behavior of the user across the web on sites that have Facebook pixel or Facebook social plugin. |
| Cookie | Duration | Description |
|---|---|---|
| _hjAbsoluteSessionInProgress | 30 minutes | No description |
| _hjid | 1 year | This cookie is set by Hotjar. This cookie is set when the customer first lands on a page with the Hotjar script. It is used to persist the random user ID, unique to that site on the browser. This ensures that behavior in subsequent visits to the same site will be attributed to the same user ID. |
| _hjIncludedInPageviewSample | 2 minutes | No description |
| _hjTLDTest | session | No description |
